Antibody discovery primer
I hope this post will serve as a reference point for readers of later posts who feel they need a little more context on the field of antibody discovery. It will go into the basics of antibodies as molecules and therapeutics, and some of the core approaches in the field. If you work with antibodies and you are familiar with all of this feel free to skip!
What is an antibody?
Antibodies are proteins. They function as a key component in many animals immune system, helping to detect and eradicate invading organisms or rogue cancerous cells from the body. What makes antibodies particularly facinating as a class of proteins is that the set of antibody proteins we are born with are not the same as the ones we have as adults. In fact, the set of antibodies circulating in your body right now is probably significantly different from the ones you had a month ago. Especially if you have caught a bug in the intervening weeks.
Why is does this make them so interesting? Well, the vast majority of proteins in our bodies are encoded by genes which do not change throughout our life. Their underlying genetic sequence is set in stone at the moment our parents genomes recombine to make ours. Antibodies are different. They can evolve and change during our lifetime in response to our environment.
This evolution results in a dizzying number of unique antibody molecules in typical adult, with estimates ranging from tens of millions to well into the billions. The human body acheives this remarkable feat using a bag of molecular tricks. For example, it mixes and matches elements from different gene cassetes resulting in large number of possible combinations. Once these gene combinations have been formed, the body can introduce additional variation using targeted mutations to part of the antibody genes.
The upshot of all this variation is that our antibody repertoires are likely to contain variants that will recognise almost any novel entity nature throws at us. When a new virus emerges that the human immune system has never encountered before, chances are we have an antibody that will be able to counter it. Not only that, but our immune system will actually generate new antibodies in response this new virus, which will be even better at recognising and neutralizing it.
This is by no means an exhaustive account of antibodies, but I hope it gives you a flavour as to why they are such interesting and important molecules.
Antibodies as therapeutics
Antibodies have been central to medical advances for centuries. Any time you get a vaccine for a disease, the result is that your body produces antibodies that recognize some piece of the disease causing pathogen. These antibodies are what provide us long term immunity to disease. However with the growth of industrial biotechnology, it was recognized that one could isolate the gene encoding a particular antibody through molecular cloning, and mass produce it.
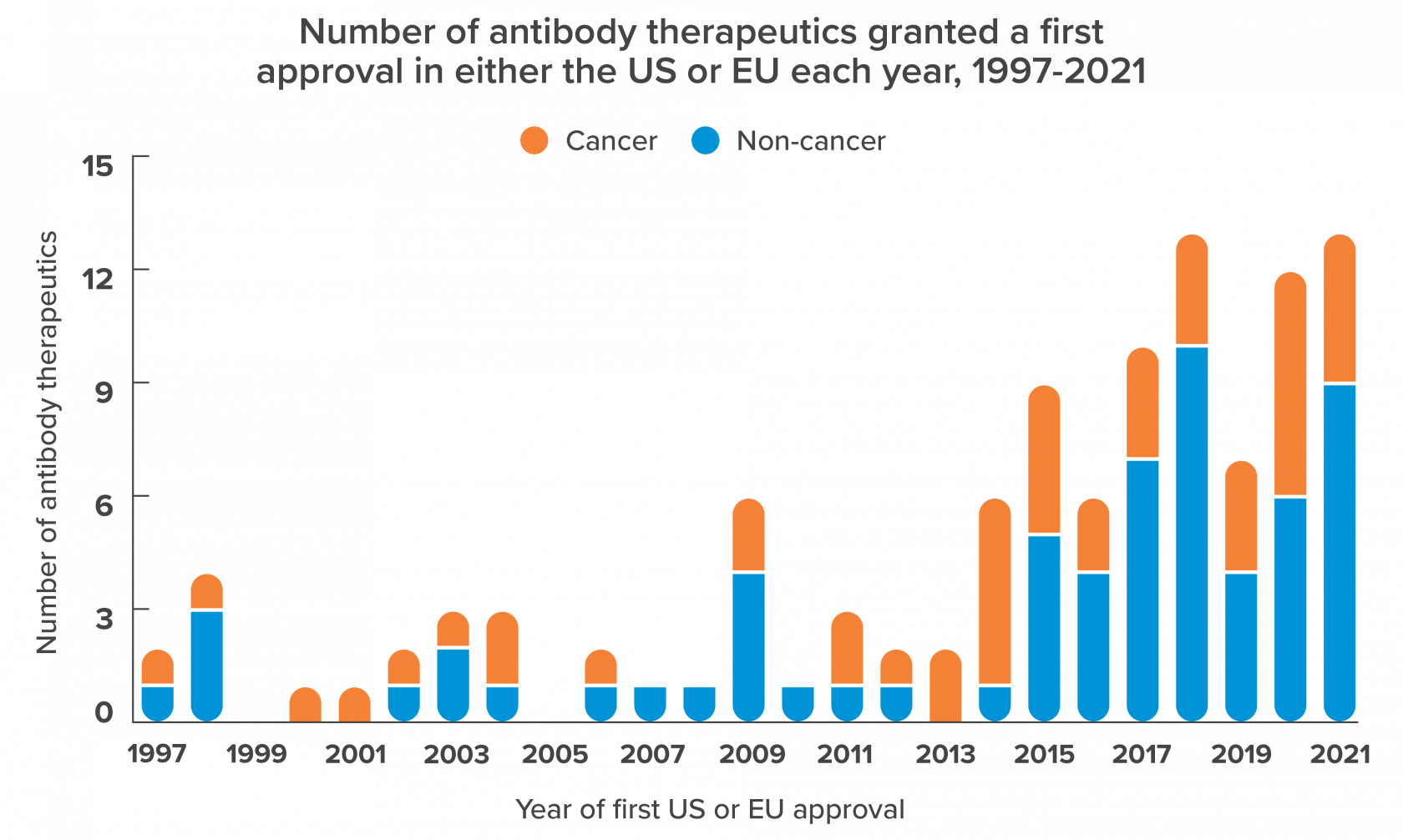
These cloned antibodies (known as recombinant antibodies) have found a wide variety of uses in the medical field. Antibodies are key to how pregnancy tests work for example, which use an antibody recognizing human chorionic gonadotropin - a hormone released in large amounts in pregnant women. The arguably more transformative application of recombinant antibodies however is as therapeutics (i.e. drugs). Once produced in large amounts, antibodies can be injected back into patients to combat disease.
As you can see from the figure on the left the approvals of antibodies as therapeutics increased dramatically in the 2010s and has continued to rise since then, with over 200 antibodies approved for therapeutic use or in regulatory review.
So whats behind this impressive adoption? Well there are several attributes of antibodies that set them apart from the traditional class of molecules used as therapeutics (small molecules), and which make them ideal drug candidates. For one thing, their large size means they interact with their targets over a wide surface area. This means that the interaction is more likely to be unique (as there are more potential permutations of interacting groups) which solves a key challenge for thereapeutics - specificity. We want therapeutics to interact with their target and nothing else. This off-target interaction as its known, is a killer of therapeutic programs, and is one of the major sources of safety issues. Antibody therapeutics promise to reduce this risk.
In addition to the specificity bonus, antibodies come with a set of built-in immune system engager functions (they are immune proteins after all!). Through these elements, they are able to recruit and activate immune cells that are able to then kill the cell the antibody is attached to, a process known as antibody dependent celullar cytotoxicity (ADCC).
Finally, the specificity of antibody therapeutics can be used to target the delivery of other drugs to particular locations in the body. Think heat seeking missile! By conjugating a toxin to an antibody the binds preferentially to cancerous cell proteins for example, we can limit the damage done to other tissues in the body.
How are antibody therapeutics discovered?
We have learned in previous sections about some of the salient features of antibodies, and why their popularity as therapeutics has taken off. But what are some of the ways they are actually discovered in practice. Well, the answer is there are many! And the makeup of these approaches has been changing over the years. Lets get into some of them.
The very first class of approaches to discovering therapeutic antibodies involved injecting an animal (typically a mouse) with your drug target, and allowing the animal to develop an immune response to the target (including target specific antibodies). The B cells (the cell type that produces antibodies) of the animal would then be harvested and screened to identify those producing target specific antibodies. The hits would be fused with myeloma cells (an immortalized B cell specialized for antibody production) from which these antibodies could be expanded and mass produced. This approach is referred to as hybridoma technology.
A later development were the so called in-vitro techniques (so called to distinguish them from the in-vivo techniques described in the last paragraph). This involved isolating antibody genes from a collection of human donors, cloning them into a new vector which can be used to express the antibody protein on the surface of a bacteriophage particle. The resulting population of antibody expressing bacteriophage is known as a library. The foundational methods using this approach were therefore named phage display.

The process of extracting target specific antibodies from antibody libraries is often refered to as biopanning or selection. A good analogy for how this works is angling. In this analogy our antibody library would represent a body of water containing fish (antibodies). We attach bait (our drug target) to a hook (biotin) and throw it into the water. After waiting a sufficient amount of time for fish to bite, we retract our baited hook using our fishing line (magnetic bead conjugated streptavidin) and land a fish (antibodies). This is obviously done millions of times at a microscopic scale, but the principle is remarkably similar!
These two approaches each come with their own strengths, weaknesses and ethical issues. For example, antibodies discovered using mice have to undergo a process known as “humanization” so that they can more safely be injected into humans without eliciting their own immune response, a process which is costly and prone to failure. However antibodies discovered using phage display do not go through the same kind of quality checks that occur inside the body during an immune response, and are therefore thought to be more prone to issues that prevent their ascension to approved therapeutic, often termed developability issues in the industry.
New variants of these techniques are being developed to address these shortcomings. For example, new transgenic mice have been developed that express human antibody genes, obviating the need for humanization. Similarly, new variants of in-vitro techniques have emerged which promise to minimize some of their associated issues. Mammalian display was developed by some of my old colleagues at Iontas is an effort to reintroduce some of the intrinsic quality filters present in in-vivo methods.
A third and much newer approach to discovering antibody therapeutics is the fully in-silico approach, where computational techniques are used to design new antibodies de-novo. The kinds of techniques employed here are diverse and growing. One example generates populations of antibody structures using combinations of existing loop fragments. These structures are then docked against the target and scored using an energy function. Successful designs are subjected to mutagenesis in an effort to improve binding energy. In-silico approaches are definately in their infancy (and will surely be the topic of many future blog posts). Difficulties remain around the ability to accurately model key components of antibody structures (namely the complementarity determining regions), limiting the applicability of these approaches. However the arrival of powerful new machine learning algorithms, combined with the ever increasing size of structural data available to learn from may ultimately knock down these barriers completely.
Conclusion
This post has glossed over many important and interesting facets of antibodies, however it has hopefully given you an overview of the field that will enable you to engage with some of the other content in this blog more easily. I also hope I have convinced you that antibody therapeutics hold huge promise! For those who want to learn more, I have included some relevant resources below.
- The Antibody Society website is a good place for news and resources relevant to those involved in antibody discovery
- mAbs is a well respected journal in the antibody field. Check out their most read section for helpful reviews
- Janeways Immunobiology is the preeminent textbook in the field of immunology and has a good section an antibodies specifically
- Antibody Drug Discovery is a more focused text, and goes into much more detail on many of the topics discussed in this post.